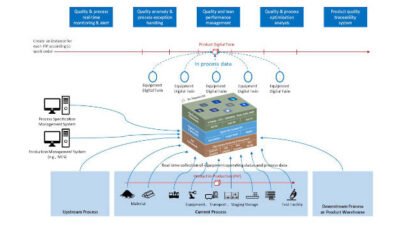
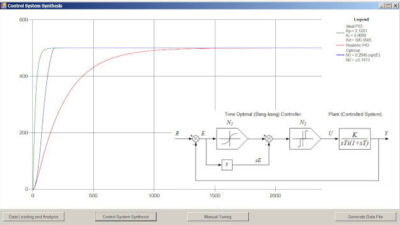
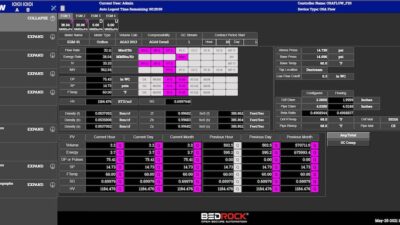
The Purdue Innovates Office licensed CrySyst to commercialize CryMoCo and CrySiV, with implications for monitoring, modeling and process control technologies.
Crystallization Systems Technology Inc. (CrySyst), a technology-focused startup, has been launched to improve crystallization processes in the pharmaceutical and fine chemical industries. The move has implications for process monitoring, modeling and process control techniques.
CrySyst’s quality-by-control (QbC) framework focuses on crystallization monitoring, modeling, and control. It is based on research published in Crystal Growth & Design (April 15, 2020; Oct. 5, 2021) and Industrial & Engineering Chemistry Research (Sept. 22, 2022).
Zoltán Nagy, the Arvind Varma Professor of Chemical Engineering in Purdue University’s Davidson School of Chemical Engineering, and Botond Szilágyi, a former postdoctoral research associate at Purdue and now an associate professor at Budapest University of Technology and Economics, founded CrySyst.
The Purdue Innovates Office of Technology Commercialization has issued a license for CrySyst to commercialize copyright technologies CryMoCo and CrySIV.
CrySiV and CryMoCo software solutions
Nagy said key challenges in model-based process development include determining necessary experiments, selecting appropriate model structures, and obtaining reliable model parameters.
CrySiV and CryMoCo provide a structured method for crystallization process development.
CryMoCo is instrument vendor-independent crystallization process monitoring and control software. It implements industry-standard communication protocols with typical process analytical technology tools and state-of-the-art process control methods, including direct nucleation control and supersaturation control approaches.
CrySiV is a simulation tool designed for crystallization process modeling. It includes kinetic parameter regression, process simulation, and visualization.
CryMoCo and CrySiV provide:
- A structured interface designed to simplify use for industry professionals.
- Advanced numerical solvers that support reliable and reproducible solutions, allowing for model validation.
- A structured workflow that assists with model selection and refinement while addressing computational uncertainties.
Challenges in process development
Pharmaceutical and fine chemical companies must develop processes with limited material, restricted experimental resources, and long optimization timelines when relying solely on experimental methods.
“These challenges can be effectively addressed through model-based digital design and model-free direct design approaches, which have seen increasing adoption,” he said. “However, to achieve widespread implementation, there is a critical need for systematic workflows and robust tools to support their integration into industrial practice. This is what CrySyst solutions address.”
Nagy and Szilágyi received funding from the Enabling Technologies Consortium.
Edited by Puja Mitra, WTWH Media, for Control Engineering, from a Purdue University news release.